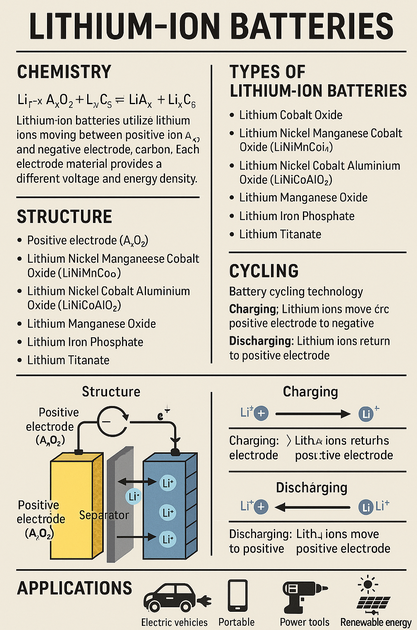
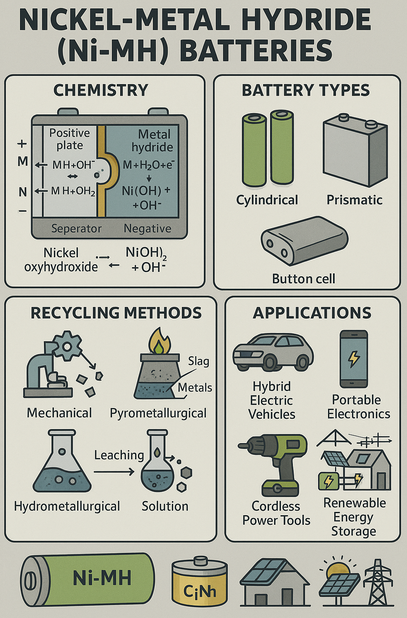
Lead-acid batteries (LABs), are the oldest type of rechargeable battery still widely used today. Known for their low cost, robustness, and high surge current capabilities, LABs are a critical component in energy storage, automotive applications, and backup power systems. With growing attention to environmental sustainability, the recycling and clean recovery of LABs have become equally important.
Chemistry of Lead-Acid Batteries
-
Electrochemical Reactions
Lead-acid batteries operate based on the reversible electrochemical reaction between lead (Pb), lead dioxide (PbO₂), and sulfuric acid (H₂SO₄):
- Discharge Reaction: PbO₂ + Pb + 2H₂SO₄ → 2PbSO₄ + 2H₂O
- Charge Reaction: 2PbSO₄ + H₂O → PbO₂ + Pb + 2H₂SO₄
-
Components
- Positive Electrode (cathode during discharge): Lead dioxide (PbO₂)
- Negative Electrode (anode during discharge): Sponge lead (Pb)
- Electrolyte: Dilute sulfuric acid (H₂SO₄)
- Separator: Microporous material preventing short circuit
Types of Lead-Acid Batteries
- Traditional design with liquid electrolyte
- Requires periodic maintenance and water refilling
- Starting, Lighting, and Ignition (SLI) Batteries: High-current bursts for engine starting, supports vehicle electrical systems, used in cars, motorcycles, and trucks
- Industrial Batteries: Stationary Batteries used in UPS, telecom, emergency lighting, substations, and Traction Batteries Used in forklifts, pallet trucks, electric buses
- Sealed, maintenance-free, and spill-proof
- Electrolyte immobilized (absorbed or gelled)
- Pressure relief valves ensure safe operation
- Absorbed Glass Mat (AGM): Used in start-stop systems, UPS, motorcycles
- Gel Cell: Suitable for solar systems, wheelchairs, marine use
Flooded (Vented) Lead-Acid Batteries
Valve-Regulated Lead-Acid (VRLA) Batteries
Structural Design and Construction
- Plates: Lead alloy grids with active material
- Separators: Prevent short circuits
- Container: Usually polypropylene
- VRLA Valves:Control internal pressure and gas release
Cycling Technology and Performance
-
Charge and Discharge Cycles
- Standard cycle life ranges from 500–1000 cycles
- VRLA batteries often have better deep discharge performance
- Charge efficiency: ~70–85% depending on type and depth of discharge (DoD)
-
Sulfation
- Reversible formation of PbSO₄ crystals during discharge
- Chronic sulfation reduces capacity—mitigated by periodic equalization or carbon additives
-
Recycling Enhancements:
- Advanced designs incorporate additives like barium sulfate or carbon
- Use of refined secondary lead improves cycling consistency
Applications
- Automotive: Starter batteries (SLI), Electric vehicles (limited by weight and energy density)
- Energy Storage: Solar PV and off-grid systems, Uninterruptible Power Supplies (UPS)
- Industrial: Forklifts, telecom towers, and emergency systems
Recycling and Environmental Aspects
Lead-acid batteries are over 95% recyclable, making them one of the most recycled consumer products globally.
-
Standard Recycling Process
- Breaking and Separation
- Desulfurization and Paste Processing
- Smelting and Refining
- Lead Recovery
-
Emerging Recycling Technologies
- Hydrometallurgy: Uses leaching and precipitation
- Electrohydrometallurgy: Electrolysis in alkaline/acidic solutions
- Vacuum Reduction: Pb-Sb alloy separation
- Electrowinning: Direct recovery from leachate
- Alkaline Conversion: For cleaner lead oxide production